Article originally published in German by TRANSLATE partner Technische Universität Darmstadt on 29th February 2024. Based on TRANSLATE’s latest publication “Giant thermoelectric response of confined electrolytes with thermally activated charge carrier generation”.
Researchers from the Institute of Nano- and Microfluidics at TU Darmstadt have discovered a mechanism that converts low-temperature waste heat into electricity. The role that salts and nanotechnology play in this process is now described by TU Professor Steffen Hardt and his colleague Dr. Rajkumar Sarma in the journal Physical Review Letters.

Waste heat from industrial plants, data centres and buildings, refrigerators, smartphones and other electronic devices is a previously underused source of clean power generation. The problem is that we cannot efficiently utilise waste heat that is less than 100 degrees Celsius. This could change in the future. Steffen Hardt, professor in the Department of Mechanical Engineering at TU Darmstadt and head of the Institute of Nano- and Microfluidics, together with his colleague Dr. Rajkumar Sarma, have identified a new mechanism that converts heat into electrical energy. In the current issue of Physical Review Letters, the two researchers describe this process.
The novel energy conversion takes place in a material with tiny nanochannels, filled with a highly concentrated salt solution. If one side of the material is warmer than the other, a thermoelectric effect occurs. Even a low temperature gradient causes an electrical voltage in the nanochannels that is much more pronounced than the established theory would suggest. Hardt says: “With our model calculations now presented, we can explain the extraordinarily high electric voltage that has been detected in some experiments.”
“The experiments with cellulose were the most promising.”
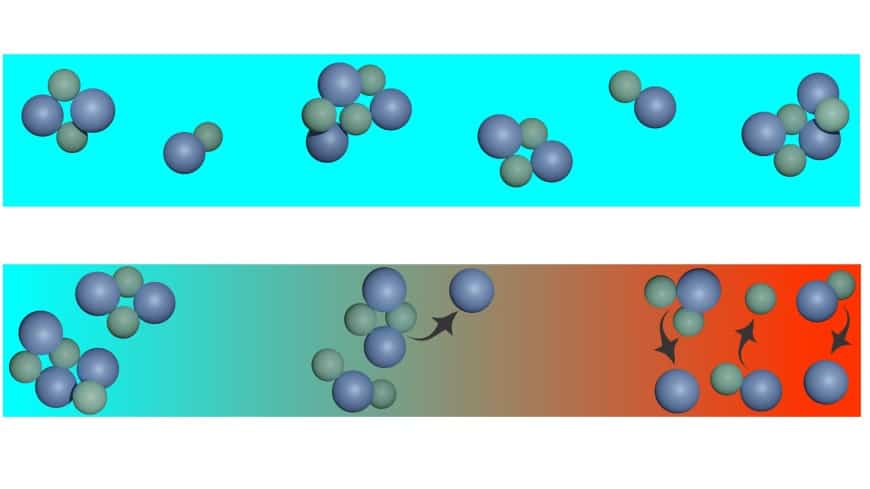
In the narrow channel with the highly concentrated salt solution, the positive and negative ions of the salt partly float around freely, partly forming charge-neutral clusters. Clustering depends on temperature. This is exactly what we can use to convert heat into electricity, because if the nanomaterial filled with the salt solution is heated on one side, the clusters there disintegrate. The released charges migrate to the colder side to compensate for the concentration gradient. This charge transport through the nanochannel is accompanied by a high electrical voltage.
Promising results with cellulose
The Darmstadt scientists are investigating such phenomena purely theoretically. However, they are cooperating with experimental research groups within the framework of the EU project Translate. For example, a team from University College Cork has investigated the effect both in a material made of oxidised aluminum and in a substance based on cellulose, the main component of plant cell walls. Both materials possess the nanochannels that are crucial for energy conversion. “The experiments with cellulose were the most promising,” says Hardt. As a renewable and abundantly available raw material, the natural material offers many advantages. But for Hardt and Sarma, it is a challenge due to its disorderly structure. The TU researchers are now expanding their theoretical model to bring it into line with the results from Cork.
Hardt cannot yet estimate when the technology will be ready for the market: “The principle works, but we have to increase the efficiency, and whether this succeeds depends above all on the material design.” However, the researchers have no shortage of ideas for application. For example, they have in mind a façade cladding made of a nanomaterial that converts part of a building’s waste heat into electricity. This is still a bold vision, but we should pursue this, because currently an estimated 70 percent of the energy produced by power plants and used by households and industry is distributed as waste heat in the atmosphere. We can no longer afford this waste.
Original article by Uta Neubauer (TUD) available here.
Read the full preprint of TRANSLATE’s latest publication here.
Related read: Nanochannel Theory Explained


