The TRANSLATE project’s overarching objective is the development of a nanofluidic platform for efficiently converting waste heat into electricity. While the project encompasses various facets, including electrode development and ion intercalation, the specific focus of Work Package 2, Task 2.2, centres on the functionalisation of nanochannel membranes to establish a charged surface and modify the charge densities on the inner surfaces of their walls.
The project’s success hinges on the control of two important factors for nanochannel-based energy harvesting – permeability and selectivity. Charged nanochannels will attract counter ions and repel co-ions leading to the creation of an electric double layer (EDL) at the channel walls. Moreover, EDL overlap within the nanochannels, is a critical element for the separation of ions and the effective conversion of waste heat into electricity. This electric double layer, formed by a charged surface on the nanochannel walls, facilitates the selective movement of ions, thereby enhancing the thermovoltage.
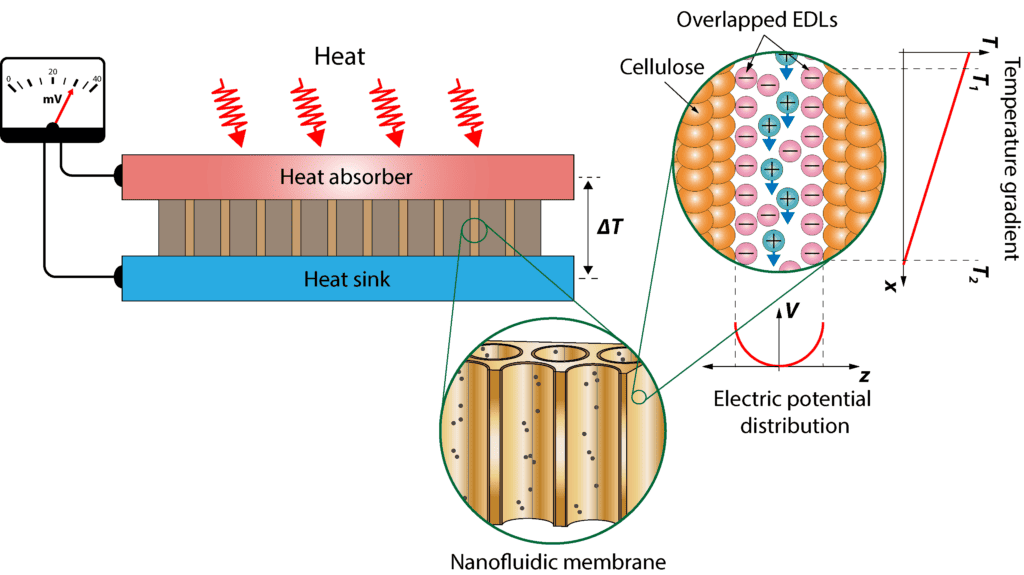
Coating the inner walls with organic ligands of different functional groups and charges leads to the formation of the electric double layer, and the attraction of oppositely charged ions. For example, a negatively charged wall selectively attracts positive ions while repelling negative ones, leading to a charge separation that is a key factor for energy harvesting culminating in what we term an “ion-selective membrane.” This ion-selective membrane allows the passage of one type of ion, augmenting ion flow and creating a higher voltage difference under temperature variation. This ion selectivity is integral to our project’s efficiency.
Achieving the necessary overlap of the electric double layer is crucial for the project’s success, with the nanochannel diameter serving as a pivotal factor. Narrow nanopore diameters play a significant role in attaining the desired electric double layer overlap, allowing for the generation of high thermovoltages. The assessment of charged surfaces and their interaction with surrounding ions in the solution involves measuring Zeta potential values, specifically at a defined plane within the electric double layer.

My work over the past two years at University College Cork (UCC) has been devoted to this crucial aspect of the project – functionalisation of the nanochannel membranes. My primary role involves the production of charged nanofluidic membranes. To make the membranes more sustainable, I have utilised materials such as anodised aluminium oxide (AAO) in collaboration with the University of Latvia and explored polycarbonate and cellulose membranes with fellow TRANSLATE team member Anjali Ashokan. Work on TRANSLATE also extends to the development of nanofluidic membranes, where I have explored materials such as graphite and polyelectrolytes to create films with incorporated nanochannels, deepening our comprehension of their properties. The significance of my work lies in contributing to the creation of functionalised nanochannel membranes. Notably, the functionalisation process has a modest yet valuable effect on pore size, contributing to their narrowing and ion selectivity via enhanced charge density. As stated above, this phenomenon enhances ionic conductivity, promotes electric double layer overlap, and increases the thermovoltage.
The long-term stability and performance of the device depends on a range of factors, including the materials used throughout the device, electrode selection, and membrane characteristics. While ion separation and ionic movement are essential for large thermovoltages , the device’s longevity and stability are contingent on the overall design and materials selected.

Our estimated progress in Task 2.2 stands at approximately 90%, with the successful functionalisation of nanochannel membranes marking a significant achievement. We are actively exploring the impact of functionalisation on ionic conductivity and thermoelectric behaviour. This phase is currently underway, with numerous samples generated and ongoing collaboration with fellow researchers. Our remaining efforts are focused on studying the effects of functionalisation on ionic conductivity, thermoelectric generation, and the selection of optimal electrodes for the device.
Presently, I am actively preparing and dispatching a comprehensive set of functionalised samples for subsequent tasks within the work packages. Our extensive sample collection includes a particular emphasis on those with the narrowest pore diameters, with ongoing exploration of even smaller sizes. Our collaborators in the University of Latvia are diligently working on further reducing pore sizes through atomic layer deposition.
I am deeply appreciative of the opportunity to collaborate with an exceptional team on the TRANSLATE project, and our collaborative spirit is commendable. I extend my gratitude to Professor Holmes for his impactful guidance. I am enthusiastic about our progress to date and eager to achieve even more in the future.

